CAR T Cell Therapy 101
Let’s take a minute to give props to your immune system.
If you’re not exactly sure what your immune system does, think of it like the mama bear of your body. The cells, tissues and organs that make up your immune system fiercely protect your body against threats (germs, viruses, etc.) and attack anything that’s potentially harmful.
Your immune system can also be a powerful weapon against cancer, thanks to a treatment option called immunotherapy. The treatments involve boosting or changing how the immune system works so it can find and destroy cancer cells.
There are different types of immunotherapy depending on the condition and diagnosis. One of the most innovative options out there is called chimeric antigen receptor (CAR) T-cell therapy, which is typically used after other therapies have been tried first.
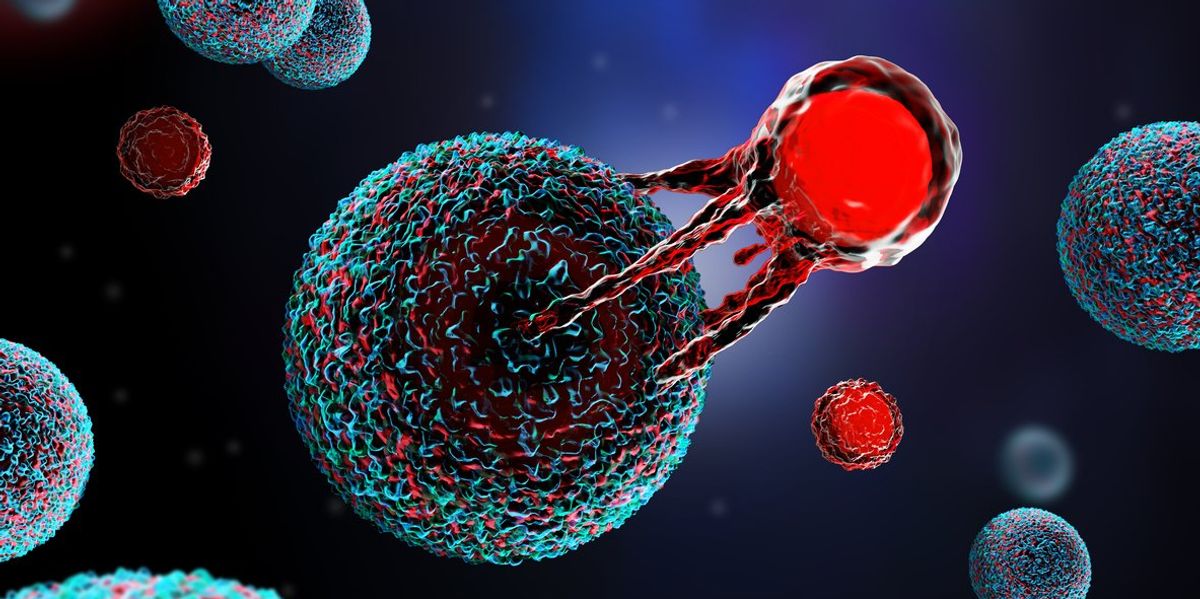
The treatment is unique compared to other cancer treatments because it uses the body’s T cells — a type of white blood cell — to help fight against the cancer.
CAR T-cell therapy isn’t an option for all cancers, but it can help people with certain blood cancers — even advanced cases of blood cancer — achieve “no evidence of disease” (a term used when cancer can no longer be detected in the body).
Read: Gene & Cell Therapy 101 >>
Here’s more on CAR T-cell therapy.
What is CAR T-cell therapy?
Chimeric antigen receptor (CAR) T-cell therapy is a type of immunotherapy created from the living T cells of the person with cancer.
The T cells are a type of white blood cell and the main slayers of disease and infected cells. The natural T cells in people with cancer aren’t the best at finding and fighting cancer cells, so CAR T-cell therapy alters the T cells in a lab so they can better search and destroy the cancer.
How CAR T-cell therapy works
CAR T-cell therapy requires a few different steps. First, blood is collected from the patient via two IV lines — one to remove the blood and separate the white blood cells — and one to put the blood back into the body.
From there, T cells are separated from other white blood cells and genetically engineered in a lab. Special genes called chimeric antigen receptors (CARs) — hence the name — are added to the surface of the T cells.
After the T cells are modified, they’re grown and multiplied to create hundreds of millions of cells that are infused into the person with cancer.
The modified cells are then able to connect to antigen proteins on cancer cells and attack the cancer.
CAR T-cell prep and infusion
The process from the first blood collection to infusion can take up to five weeks.
In the weeks before the infusion, some people may have chemotherapy to weaken the immune system to give the modified T cells a better chance to fight the cancer.
When it’s time, CAR T-cell therapy is given in a single infusion via IV — a process that usually lasts around an hour.
CAR T-cell therapy side effects
Like all cancer treatments, CAR T-cell therapy can have a range of side effects, some of which can be severe, including infection and the death of B cells, which produce antibodies.
The good news is that research shows that the benefits of these treatments outweigh their risks. As a result, the Food and Drug Administration (FDA) has removed the Risk Evaluation and Mitigation Strategy (REMS) requirements, which are protocols that are put in place to manage risks, from the approved CAR T-cell immunotherapies.
When the CAR T cells multiply, they can release chemicals in the blood (cytokines) that can ramp up the immune system and cause possibly life-threatening issues. This is called cytokine release syndrome (CRS).
The side effects of CRS can include:
- High fever and chills
- Breathing problems
- Dizziness
- Fatigue
- Headache attacks
- Joint pain
- Muscle pain
- Nausea, vomiting and diarrhea
- Rapid heartbeat
CAR T-cell therapy may also affect the nervous system and lead to immune effector cell-associated neurotoxicity syndrome (ICANS). The symptoms of the condition can include:
- Confusion
- Changes in consciousness
- Headache attacks
- Loss of balance
- Tremors
- Trouble speaking or understanding
- Seizures
Other side effects of CAR T-cell therapy can include:
- Low levels of minerals in the blood
- Allergic reaction during the infusion
- Weakened immune system
- Low blood cell counts
- Increased risk for other blood cancers
What are CAR T-cell therapies approved for?
CAR T-cell therapies were first approved by the FDA in 2017 for children with acute lymphoblastic leukemia. Today, CAR T-cell therapy is also approved to treat adults.
In addition to acute lymphoblastic leukemia, CAR T-cell therapy can be used to treat other types of blood cancer. These can include:
- Multiple myeloma
- High-grade B-cell lymphoma
- Follicular lymphoma
- Primary mediastinal large B-cell lymphoma
- Mantle cell lymphoma
Given the success with blood cancers, a number of studies are now looking at CAR T-cell therapy for solid tumor cancers, such as brain, pancreatic, colorectal and triple negative breast cancer, as well as for autoimmune diseases (e.g., lupus, multiple sclerosis), cardiac and liver fibrosis, HIV, and Type 1 diabetes.
If I have cancer, how do I know if I’m eligible for CAR T-cell therapy?
CAR T-cell therapies are used to help treat blood cancer that has returned (relapsed) or blood cancer that hasn’t responded to previous treatments. If you have blood cancer, you should talk to your HCP to see if you’re a candidate.
Does insurance cover CAR T-cell therapy?
Many insurance plans cover CAR T-cell therapy, but the cost of treatment varies depending on the plan, and prior authorization may be required.
Medicare covers FDA-approved CAR T-cell therapy, and Medicaid may also cover it. Coverage can vary depending on the state.
For people who don’t have insurance or need financial assistance, CAR T-cell treatment centers and medication manufacturers may help with treatment costs and travel expenses like transportation, meals and lodging. Nonprofit organizations like The Leukemia & Lymphoma Society may offer financial support options too.
Access to CAR T-cell therapy
When the FDA removed the REMS requirement for six approved CAR T-cell therapies last month, the move also made the potentially life-saving treatments easier to access.
Without the REMS requirement, hospitals and clinics no longer need to have a certification to administer the treatments.
Also good to note: The FDA cut down the time people must wait to drive after treatment from eight weeks to two weeks. And the FDA shortened the requirement to stay near a healthcare facility from four weeks to two weeks.
These changes help offer more flexibility and opportunities for treatment.
If you or someone you know is interested in CAR T-cell therapy, talk to an oncologist about the facts, including where to go for treatment.
This educational resource was created with support from Bristol Myers Squibb, a HealthyWomen Corporate Advisory Council member.
From Your Site Articles
Related Articles Around the Web
Source link
Share this article: