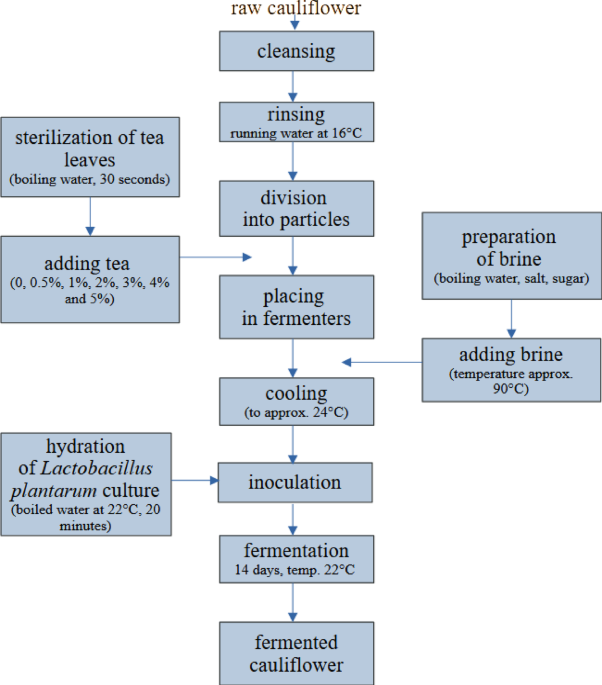
Application of green tea to enhance the antioxidant properties of fermented cauliflower
Jarmoluk, A. Food technology. T.1. Raw materials, products, functional food (in Polish) (Educational Publishing House WSiP, 2016).
Engel, E., Baty, C., Le Corre, D., Souchon, I. & Martin, N. Flavor-active compounds potentially implicated in cooked cauliflower acceptance. J. Agric. Food Chem. 50, 6459–6467. https://doi.org/10.1021/jf025579u (2002).
Bell, L., Oloyede, O. O., Lignou, S., Wagstaff, C. & Methven, L. Taste and flavor perceptions of glucosinolates, isothiocyanates, and related compounds. Mol. Nutr. Food Res. 62, 1700990. https://doi.org/10.1002/mnfr.201700990 (2018).
Wieczorek, M. N. et al. The relation between phytochemical composition and sensory traits of selected Brassica vegetables. LWT 156, 113028. https://doi.org/10.1016/j.lwt.2021.113028 (2022).
Thompson, H. O., Önning, G., Holmgren, K., Strandler, H. S. & Hultberg, M. Fermentation of cauliflower and white beans with Lactobacillus plantarum—Impact on levels of riboflavin, folate, vitamin B12, and amino acid composition. Plant Foods Hum. Nutr. 75, 236–242. https://doi.org/10.1007/s11130-020-00806-2 (2020).
Jastrzębska, A. et al. Determination of selected biogenic amines in fermented vegetables juices. Food Control 154, 109980. https://doi.org/10.1016/j.foodcont.2023.109980 (2023).
Paramithiotis, S., Hondrodimou, O. L. & Drosinos, E. H. Development of the microbial community during spontaneous cauliflower fermentation. Food Res. Int. 43, 1098–1103. https://doi.org/10.1016/j.foodres.2010.01.023 (2010).
Rachwał, K., Gustaw, K. & Sadok, I. Enhancing food sustainability through probiotics isolated from fermented cauliflower. Sustainability 16, 8340. https://doi.org/10.3390/su16198340 (2024).
Herman, A., Matulewicz, O., Korzeniowska, E. & Herman, A. P. Determination of post-fermentation waste from fermented vegetables as potential substitutes for preservatives in o/w emulsion. Int. J. Mol. Sci. 25, 5510. https://doi.org/10.3390/ijms25105510 (2024).
Xu, H., Wu, H., Zhou, R., Yu, F. & Zang, R. The effects of fermented cauliflower residue feed on the diarrhea rate, intestinal morphology, immune indicators, and intestinal flora of weaned piglets. Fermentation 10, 465. https://doi.org/10.3390/fermentation10090465 (2024).
Wojdyła, T. & Wichrowska, D. The influence of additives used and storage methods on the quality of sauerkraut. Inż. Ap. Chem. 53, 424–426 (2014).
Choi, W.-Y. & Park, K.-Y. Increased preservative and antimutagenic activities of kimchi with addition of green tea leaves. J. Food Sci. Nat. 5, 189–193 (2000).
Patent KR100384309B1. Functional Kimchi added green tea and process for preparation thereof (Korea, 2003).
Patent KR20170025806A. Kimchi Composition Comprising Vitamin C and Its Antioxidative Activity and Whitening Effect (Korea, 2017).
Musial, C., Kuban-Jankowska, A. & Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 21, 1744. https://doi.org/10.3390/ijms21051744 (2020).
Cichoń, Z., Miśniakiewicz, M. & Szkudlarek, E. Properties of green tea (in Polish). Zesz. Nauk. 73, 59–90 (2007).
Kodama, D. H., Goncalves, A. E. S. S., Lajolo, F. M. & Genovese, M. I. Flavonoids, total phenolics and antioxidant capacity: Comparison between commercial green tea preparations. Ciênc. Tecnol. Aliment. Camp. 30, 1077–1082. https://doi.org/10.1590/S0101-20612010000400037 (2010).
Luo, Q. et al. Green extraction of antioxidant polyphenols from green tea (Camellia sinensis). Antioxidants 9, 785. https://doi.org/10.3390/antiox9090785 (2020).
Narukawa, M. et al. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem. Biophys. Res. Commun. 405, 620–625. https://doi.org/10.1016/j.bbrc.2011.01.079 (2011).
Han, T. & Aye, K. N. The legend of laphet: A Myanmar fermented tea leaf. J. Ethn. Foods 2, 173–178. https://doi.org/10.1016/j.jef.2015.11.003 (2015).
Cao, L. T. et al. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. J. Food Drug Anal. 26, 112–123. https://doi.org/10.1016/j.jfda.2016.11.020 (2018).
Zhao, Z.-J. et al. Flavour chemical dynamics during fermentation of kombucha tea. EJFA 30, 732–741. https://doi.org/10.9755/ejfa.2018.v30.i9.1794 (2018).
Cheng, L. et al. Distinct changes of metabolic profile and sensory quality during Qingzhuan tea processing revealed by LC-MS-Based metabolomics. J. Agri. Food Chem. 68, 4955–4965. https://doi.org/10.1021/acs.jafc.0c00581 (2020).
Wang, R., Sun, J. C., Lassabliere, B., Yu, B. & Liu, S. Q. UPLC-Q-TOF-MS based metabolomics and chemometric analyses for green tea fermented with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. Curr. Res. Food Sci. 5, 471–478. https://doi.org/10.1016/j.crfs.2022.02.012 (2022).
Bogdański, P. et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr. Res. 32, 421–427. https://doi.org/10.1016/j.nutres.2012.05.007 (2012).
Indarti, K., Apriani, E. F., Wibowo, A. E. & Simanjuntak, P. Antioxidant activity of ethanolic extract and various fractions from green tea (Camellia sinensis L.) leaves. Pharmacog. J. 11, 771–776. https://doi.org/10.5530/pj.2019.11.122 (2019).
Xu, X. Y. et al. Effects and mechanisms of tea for the prevention and management of cancers: An updated review. Crit. Rev. Food Sci. Nutr. 60, 1693–1705. https://doi.org/10.1080/10408398.2019.1588223 (2020).
Hirose, K. et al. Preparing and characterizing of xyloglucan films containing tea extract for oral mucositis. ACS Omega 10, 390–399. https://doi.org/10.1021/acsomega.4c06410 (2025).
Liu, S., Zhang, Q., Li, H., Qiu, Z. & Yu, Y. Comparative assessment of the antibacterial efficacies and mechanisms of different tea extracts. Foods 11, 620. https://doi.org/10.3390/foods11040620 (2022).
Alkufeidy, R. M., Altuwijri, L. A., Aldosari, N. S., Alsakabi, N. & Dawoud, T. M. Antimicrobial and synergistic properties of green tea catechins against microbial pathogens. J. King Saud Univ. Sci. 36, 103277. https://doi.org/10.1016/j.jksus.2024.103277 (2024).
Parvez, Md. A. K. et al. Antibacterial activities of green tea crude extracts and synergistic effects of epigallocatechingallate (EGCG) with gentamicin against MDR pathogens. Heliyon 5, e02126. https://doi.org/10.1016/j.heliyon.2019.e02126 (2019).
Kováć, J., Slobodníková, L., Nebus, B. & Kurin, E. Antibacterial activity of green tea and peppermint extracts against Enterococcus faecalis and the potential of EGCG in oral health. Eur. Pharm. J. 2025, 1–7. https://doi.org/10.2478/afpuc-2025-0002 (2025).
McCue, P. P. & Shetty, K. Phenolic antioxidant mobilization during yogurt production from soymilk using Kefir cultures. Process Biochem. 40, 1791–1797 (2005).
Lee, H. C., Jenner, A. M., Low, C. S. & Lee, Y. K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 157, 876–884 (2006).
Jaziri, I., Ben Slama, M., Mhadhbi, H., Urdaci, M. C. & Hamdi, M. Effect of green and black teas (Camellia sinensis L.) on the characteristic microflora of yogurt during fermentation and refrigerated storage. Food Chem. 112, 614–620 (2009).
Neffe-Skocińska, K., Jaworska, D., Kołożyn-Krajewska, D., Dolatowski, Z. & Jachacz-Jówko, L. The effect of LAB as probiotic starter culture and green tea extract addition on dry fermented pork loins quality. Biomed. Res. Int. 19, 452757. https://doi.org/10.1155/2015/452757 (2015).
Jin, Y. H. et al. Lactic acid fermented green tea with Levilactobacillus brevis capable of producing γ-aminobutyric acid. Fermentation 7, 110. https://doi.org/10.3390/fermentation7030110 (2021).
Wang, R., Sun, J., Lassabliere, B., Yu, B. & Liu, S. Green tea fermentation with Saccharomyces boulardii CNCM I-745 and Lactiplantibacillus plantarum 299V. LWT 157, 113081. https://doi.org/10.1016/j.lwt.2022.113081 (2024).
Nguyen, N. K., Dong, N. T., Nguyen, H. & Le, P. H. Lactic acid bacteria: Promising supplements for enhancing the biological activities of Kombucha. Springer Plus 4, 91. https://doi.org/10.1186/s40064-015-0872-3 (2015).
Xia, X.-X., Wang, B. & Fang, F. Enhancement of Kombucha fermentation by adding lactic acid bacteria. Food Ferment. Ind. 44, 185–192. https://doi.org/10.13995/j.cnki.11-1802/ts.017688 (2018).
Zhuang, X. et al. Fermentation quality of herbal tea residue and its application in fattening cattle under heat stress. BMC Vet. Res. 17, 348. https://doi.org/10.1186/s12917-021-03061-y (2021).
Chen, X., Zhou, X., Li, S., Zhang, H. & Liu., Z.,. Effects of tea residues-fermented feed on production performance, egg quality, antioxidant capacity, caecal microbiota, and ammonia emissions of laying hens. Front. Vet. Sci. 10, 1195074. https://doi.org/10.3389/fvets.2023.1195074 (2023).
Hong, G.-H., Lee, S.-Y., Yoo, J.-I., Chung, J. H. & Park, K.-Y. Catechin with lactic acid bacteria starters enhances the antiobesity effect of kimchi. J. Med. Food 26, 560–569. https://doi.org/10.1089/jmf.2023.K.0067 (2023).
Hong, G.-H., Lee, S.-Y. & Park, K.-Y. Antiobesity effect and metabolite analysis of catechin functional kimchi. J. Ethn. Foods 11, 32. https://doi.org/10.1186/s42779-024-00248-0 (2024).
Hayashi, T., Ueda, S., Suruta, H. T., Kuwahara, H. & Osawa, R. Complexing of green tea Catechins with food constituents and degradation of the complexes by Lactobacillus plantarum. BMFH 31, 27–36. https://doi.org/10.12938/bmfh.31.27 (2012).
Tarrah, A. et al. Probiotic potential and biofilm inhibitory activity of Lactobacillus casei group strains isolated from infant feces. J. Funct. Foods 54, 489–497. https://doi.org/10.1016/j.jff.2019.02.004 (2019).
Tumbarski, Y. et al. Characterization and selection of Lactobacillus strains with potential probiotic applications. Appl. Sci. 15, 2902. https://doi.org/10.3390/app15062902 (2025).
Anumudu, C. K., Miri, T. & Onyeaka, H. Multifunctional applications of lactic acid bacteria: Enhancing safety, quality, and nutritional value in foods and fermented beverages. Foods 13, 3714. https://doi.org/10.3390/foods13233714 (2024).
Bangar, S. P., Suri, S., Trif, M. & Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 46, 101615. https://doi.org/10.1016/j.fbio.2022.101615 (2022).
Ravyts, F., Vuyst, L. D. & Leroy, F. Bacterial diversity and functionalities in food fermentations. Eng. Life Sci. 12, 356–367. https://doi.org/10.1002/elsc.201100119 (2012).
Zielinski, H., Surma, M. & Zielinska, D. The naturally fermented sour pickled cucumbers. In Fermented Foods in Health and Disease Prevention (eds Frias, J. et al.) 503–516 (Academic Press, Cambridge, 2017).
Daba, G. M., Elnahas, M. O. & Elkhateeb, W. A. Contributions of exopolysaccharides from lactic acid bacteria as biotechnological tools in food, pharmaceutical, and medical applications. Int. J. Biol. Macromol. 173, 79–89. https://doi.org/10.1016/j.ijbiomac.2021.01.110 (2021).
Korcz, E. & Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 110, 375–384. https://doi.org/10.1016/j.tifs.2021.02.014 (2021).
Kuria, M. W., Matofari, J. W. & Nduko, J. M. Physicochemical, antioxidant, and sensory properties of functional mango (Mangifera indica L.) leather fermented by lactic acid bacteria. J. Agric. Food Res. 6, 100206. https://doi.org/10.1016/j.jafr.2021.100206 (2021).
Quan, Q., Liu, W., Guo, J., Ye, M. & Zhang, J. Effect of six lactic acid bacteria strains on physicochemical characteristics, antioxidant activities and sensory properties of fermented orange juices. Foods 11, 1920. https://doi.org/10.3390/foods11131920 (2022).
Xu, H. et al. Change of phytochemicals and bioactive substances in Lactobacillus fermented Citrus juice during the fermentation process. LWT 180, 114715. https://doi.org/10.1016/j.lwt.2023.114715 (2023).
Jayashree, S., Jayaraman, K. & Kalaichelvan, G. Isolation, screening and characterization of riboflavin producting lactic acid bacteria from Katpadi Vellore district. Recent Res. Sci. Technol. 2, 83–88 (2010).
Capozzi, V. et al. Biotechnological production of vitamin B2-enriched bread and pasta. J. Agric. Food Chem. 59, 8013–8020. https://doi.org/10.1021/jf201519h (2011).
Santos, F., Wegkamp, A., de Vos, W. M., Smid, E. J. & Hugenholtz, J. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 74, 3291–3294. https://doi.org/10.1128/AEM.02719-07 (2008).
LeBlanc, J. G., Taranto, M. P., Molina, V. & Sesma, F. B-group vitamins production by probiotic lactic acid bacteria in Biotechnology of Lactic Acid Bacteria: Novel Applications (eds. Mozzi, F., Raya, R., Vignolo G.) 211–232 (Wiley-Blackwell 2010).
Torres, A. C. et al. Cobalamin production by Lactobacillus coryniformis: Biochemical identification of the synthetized corrinoid and genomic analysis of the biosynthetic cluster. BMC Microbiol. 16, 240. https://doi.org/10.1186/s12866-016-0854-9 (2016).
Li, P., Gu, Q., Yang, L., Yu, Y. & Wang, Y. Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chem. 234, 494–501. https://doi.org/10.1016/j.foodchem.2017.05.037 (2017).
Singh, B. & Sharma, S. Vitamin B12 production by Lactobacillus species isolated from milk products. J. Res. Appl. Sci. Biotechnol. 1, 48–59. https://doi.org/10.55544/jrasb.1.2.6 (2022).
Morishita, T., Tamura, N., Makino, T. & Kudo, S. Production of menaquinones by lactic acid bacteria. J. Dairy Sci. 82, 1897–1903 (1999).
Boe, C. A. & Holo, H. Engineering Lactococcus lactis for Increased Vitamin K2 Production. Front Bioeng. Biotechnol. 8, 191. https://doi.org/10.3389/fbioe.2020.00191 (2020).
Behera, S. S., Ray, R. C. & Zdolec, N. Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. BioMed. Res. Int. 2018, 9361614. https://doi.org/10.1155/2018/9361614 (2018).
Tang, H., Huang, W. & Yao, Y.-F. The metabolites of lactic acid bacteria: classification, biosynthesis and modulation of gut microbiota. Microbial Cell 10, 49–62. https://doi.org/10.15698/mic2023.03.792 (2023).
Knez, E., Kadac-Czapska, K. & Grembecka, M. Fermented vegetables and legumes vs lifestyle diseases: microbiota and more. Life 13, 1044. https://doi.org/10.3390/life13041044 (2023).
Shah, A. M., Tarfeen, N., Mohamed, H. & Song, Y. Fermented foods: Their health-promoting components and potential effects on gut microbiota. Fermentation 9, 118. https://doi.org/10.3390/fermentation9020118 (2023).
Todorovic, S. et al. Health benefits and risks of fermented foods—the PIMENTO initiative. Front. Nutr. 11, 1458536. https://doi.org/10.3389/fnut.2024.1458536 (2024).
AOAC. Official Method of Analysis, 18th ed. (Association of Officiating Analytical Chemists, 2015).
Krełowska-Kułas, M. Analyses of Food Products Quality (in polish) (PWE, 1993).
Turkmen, N., Sari, F. & Velioglu, Y. S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 93, 713–718. https://doi.org/10.1016/j.foodchem.2004.12.038 (2005).
Shraim, A. M., Ahmed, T. A., Rahman, M. M. & Hijji, Y. M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 150, 111932. https://doi.org/10.1016/j.lwt.2021.111932 (2021).
Brand-Williams, W., Cuvelier, M. E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 28, 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5 (1995).
Re, R. et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3 (1999).
Benzie, I. F. F. & Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of ‘Antioxidant Power’: The FRAP Assay. Anal. Biochem. 239, 70–76. https://doi.org/10.1006/abio.1996.0292 (1996).
PN-EN ISO 8586:2014-03 Sensory analysis. General guidelines for the selection, training, and monitoring of selected assessors and sensory evaluation experts. Polish Committee for Standardization.
PN-ISO 5496:1997 Sensory analysis. Methodology. Introduction and training of assessors in the detection and recognition of odors. Polish Committee for Standardization.
PN-ISO 3972:2016-07 Sensory analysis. Methodology. Methods for testing taste sensitivity. Polish Committee for Standardization
PN-EN ISO 11132:2017-08 Sensory analysis. Methodology. Guidelines for monitoring the performance of a quantitative sensory system. Polish Committee for Standardization.
PN-EN ISO 8589:2010 Sensory analysis. General guidelines for the design of sensory analysis laboratories. Polish Committee for Standardization.
PN-ISO 5497:1998 Sensory analysis. Methodology. Guidelines for the preparation of samples for which direct sensory analysis is not possible. Polish Committee for Standardization.
PN-ISO 11035:1999 Sensory analysis. Identification and selection of descriptors for determining the sensory profile using multivariate methods. Polish Committee for Standardization.
PN-EN ISO 13299:2010 Sensory analysis—Methodology—General guidelines for determining sensory profiles. Polish Committee for Standardization.
PN-ISO 4121:1998 Sensory analysis—Methodology—Evaluation of food products using scaling methods. Polish Committee for Standardization.
PN-EN ISO 11036:1999 Sensory analysis. Methodology. Texture profiling. Polish Committee for Standardization
Migut, D., Gorzelany, J. & Wołowiec, A. Evaluation of selected chemical properties of fresh and pickled field cucumbers. Inż. Przetw. Spoż. Pol. J. Food Eng. 3, 33–39 (2018).
Kao, C.-C. & Lin, J.-Y. Culture condition optimization of naturally lacto-fermented cucumbers based on changes in detrimental and functional ingredients. Food Chem. X 19, 100839. https://doi.org/10.1016/j.fochx.2023.100839 (2023).
Kiczorowski, P., Kiczorowska, B., Samolińska, W., Szmigielski, M. & Winiarska-Mieczan, A. Effect of fermentation of chosen vegetables on the nutrient, mineral, and biocomponent profile in human and animal nutrition. Sci. Rep. 12, 13422. https://doi.org/10.1038/s41598-022-17782-z (2022).
Zhou, X. et al. Dynamic changes in physic-chemical properties and bacterial community during natural fermentation of tomatoes. Food Sci. Technol. Caminas 42, e63520. https://doi.org/10.1590/fst.63520 (2022).
Ghosh, D. Studies on the changes of biochemical, microbiological and sensory parameters of sauerkraut and fermented mix vegetables. Food Res. 5, 78–83. https://doi.org/10.26656/fr.2017.5(1).193 (2021).
Singhal, P., Satya, S. & Naik, S. N. Fermented bamboo shoots: a complete nutritional, anti-nutritional and antioxidant profile of the sustainable and functional food to food security. Food Chem. 3, 100041. https://doi.org/10.1016/j.fochms.2021.100041 (2021).
Ye, J.-H., Huang, L.-Y., Terefe, N. S. & Augustin, M. A. Fermentation-based biotransformation of glucosinolates, phenolics and sugars in retorted broccoli puree by lactic acid bacteria. Food Chem. 286, 616–623. https://doi.org/10.1016/j.foodchem.2019.02.030 (2019).
Dissanayake, I. H. et al. Lactic acid bacterial fermentation as a biotransformation strategy to enhance the bioavailability of phenolic antioxidants in fruits and vegetables: A comprehensive review. Food Res. Int. 209, 116283. https://doi.org/10.1016/j.foodres.2025.116283 (2025).
Yang, F. et al. Effects of fermentation on bioactivity and the composition of polyphenols contained in polyphenol-rich foods: A review. Foods 12(17), 3315 (2023).
Kim, G. Y. et al. Synergistic antioxidant and anti-inflammatory activities of kale juice fermented with Limosilactobacills reuteri EFEL6901 or Limosilactobacills fermentum EFEL6800. Antioxidants 12(10), 1850 (2023).
Yang, X. et al. Antioxidant properties of a vegetable–fruit beverage fermented with two Lactobacillus plantarum strains. Food Sci. Biotech. 27, 1719–1726. https://doi.org/10.1007/s10068-018-0411-4 (2018).
Park, S.B., Han, B.K., Oh, H.J., Lee, SJ, Cha, S.K., Park, Y.S., & Choi, H.J. Bioconversion of green tea extract using lactic acid bacteria. Food Eng. Prog. 16, 26–32 (2012).
Hu, T., Shi, S. & Ma, Q. Modulation effects of microorganisms on tea in fermentation. Front. Nutr. 9, 931790. https://doi.org/10.3389/fnut.2022.931790 (2022).
Lee, N.-K. & Paik, H.-D. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 27, 869–877. https://doi.org/10.4014/jmb.1612.12005 (2017).
RuizRodríguez, L. G. et al. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 140, 109854. https://doi.org/10.1016/j.foodres.2020.109854 (2021).
Cvetković, D. et al. Survival of wild strains of Lactobacilli during Kombucha fermentation and their contribution to functional characteristics of beverage. Pol. J. Food Nutr. Sci. 69, 407–415. https://doi.org/10.31883/pjfns/112276 (2019).
Nishioka, H., Ohno, T., Iwahashi, H. & Horie, M. Diversity of Lactic acid bacteria involved in the fermentation of Awa-bancha. Microbes Environ. 36, ME21029. https://doi.org/10.1264/jsme2.ME21029 (2021).
Nout, M. J. R. & Ngoddy, P. O. Technological aspects of preparing affordable fermented complementary foods. Food Control 8, 279–287 (1997).
Wang, R., Sun, J. C., Lassabliere, B., Yu, B. & Liu, S. Q. Fermentation characteristics of four non-Saccharomyces yeasts in green tea slurry. Food Microbiol. 92, 03609. https://doi.org/10.1016/j.fm.2020.103609 (2020).
Wang, R., Sun, J. C., Lassabliere, B., Yu, B. & Liu, S. Q. 13-Glucosidase activity of Cyberlindnera (Williopsis) saturnus var. mrakii NCYC 2251 and its fermentation effect on green tea aroma compounds. LWT 151, 112184. https://doi.org/10.1016/j.lwt.2021.112184 (2021).
Martinez-Villaluenga, C. et al. Influence of fermentation conditions on glucosinolates, ascorbigen, and ascorbic acid content in white cabbage (Brassica oleracea var. capitata cv. taler) cultivated in different seasons. J. Food Sci. 74, C62–C67. https://doi.org/10.1111/j.1750-3841.2008.01017.x (2009).
Ciska, E., Honke, J. & Drabińska, N. Changes in glucosinolates and their breakdown products during the fermentation of cabbage and prolonged storage of sauerkraut: focus on sauerkraut juice. Food Chem. 365, 130498. https://doi.org/10.1016/j.foodchem.2021.130498 (2021).
Yang, X. et al. Microbial community dynamics and metabolome changes during spontaneous fermentation of northeast sauerkraut from different households. Front. Microbiol. 11, 1878. https://doi.org/10.3389/fmicb.2020.01878 (2020).
Yang, X. et al. Comparison of northeast sauerkraut fermentation between single lactic acid bacteria strains and traditional fermentation. Food Res. Int. 137, 109553. https://doi.org/10.1016/j.foodres.2020.109553 (2020).
Satora, P., Skotniczny, M., Strnad, S. & Piechowicz, W. Chemical composition and sensory quality of sauerkraut produced from different cabbage varieties. LWT 136, 110325. https://doi.org/10.1016/j.lwt.2020.110325 (2021).
Major, N. et al. Bioactive properties, volatile compounds, and sensory profile of sauerkraut are dependent on cultivar choice and storage conditions. Foods 11, 1218. https://doi.org/10.3390/foods11091218 (2022).
Janiszewska-Turak, E., Kołakowska, W., Pobiega, K. & Gramza-Michałowska, A. Influence of drying type of selected fermented vegetables pomace on the natural colorants and concentration of lactic acid bacteria. Appl. Sci. 11, 7864. https://doi.org/10.3390/app11177864 (2021).
Yang, H. I. et al. Influence of salt concentration on Kimchi cabbage (Brassica rapa L. ssp. pekinensis) mass transfer kinetics and textural and microstructural properties during osmotic dehydration. J. Food Sci. 88, 1610–1622. https://doi.org/10.1111/1750-3841.16514 (2023).
Source link
Share this article: