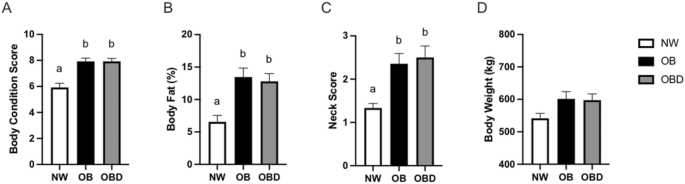
Adiposity in mares induces insulin dysregulation and mitochondrial dysfunction which can be mitigated by nutritional intervention
Giles, S. L., Rands, S. A., Nicol, C. J. & Harris, P. A. Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies. PeerJ 2, e299 (2014).
Kosolofski, H. R., Gow, S. P. & Robinson, K. A. Prevalence of obesity in the equine population of Saskatoon and surrounding area. Can. Vet. J. 58
Ertelt, A., Barton, A.-K., Schmitz, R. R. & Gehlen, H. Metabolic syndrome: Is equine disease comparable to what we know in humans?. Endocr. Connect. 3, R81–R93 (2014).
Rendle, D. et al. Equine obesity: current perspectives. UK-Vet. Equine 2, 1–19 (2018).
Johnson, P. J., Wiedmeyer, C. E., LaCarrubba, A., Ganjam, V. K. S. & Messer, N. T. Diabetes, insulin resistance, and metabolic syndrome in horses. J. Diabetes Sci. Technol. 6, 534–540 (2012).
Morgan, R. A., Keen, J. A., Walker, B. R. & Hadoke, P. W. F. Vascular dysfunction in horses with endocrinopathic laminitis. PLoS ONE 11, e0163815 (2016).
National Health Statistics Reports, Number 158, June 14, 2021 (2021).
Rashid, M. N., Fuentes, F., Touchon, R. C. & Wehner, P. S. Obesity and the risk for cardiovascular disease. Prev. Cardiol. 6, 42–47 (2003).
Wondmkun, Y. T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. DMSO 13, 3611–3616 (2020).
Aronow, W. S. Association of obesity with hypertension. Ann. Transl. Med. 5, 350–350 (2017).
Kottaisamy, C. P. D., Raj, D. S., Prasanth Kumar, V. & Sankaran, U. Experimental animal models for diabetes and its related complications—A review. Lab. Anim. Res. 37, 23 (2021).
de Mello, A. H., Costa, A. B., Engel, J. D. G. & Rezin, G. T. Mitochondrial dysfunction in obesity. Life Sci. 192, 26–32 (2018).
Schrauwen, P., Schrauwen-Hinderling, V., Hoeks, J. & Hesselink, M. K. C. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 1801, 266–271 (2010).
Siard-Altman, M. H. et al. Relationships of inflamm-aging with circulating nutrient levels, body composition, age, and pituitary pars intermedia dysfunction in a senior horse population. Vet. Immunol. Immunopathol. 221, 110013 (2020).
Sessions-Bresnahan, D. R., Heuberger, A. L. & Carnevale, E. M. Obesity in mares promotes uterine inflammation and alters embryo lipid fingerprints and homeostasis. Biol. Reprod. 99, 761–772 (2018).
Li, M. et al. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Targeted Ther. 7 (2022).
Stewart-Hunt, L., Pratt-Phillips, S., McCUTCHEON, L. J. & Geor, R. J. Dietary energy source and physical conditioning affect insulin sensitivity and skeletal muscle glucose metabolism in horses: Diet, insulin sensitivity and muscle glucose metabolism in horses. Equine Vet. J. 42, 355–360 (2010).
Kayar, S. R., Hoppeler, H., Mermod, L. & Weibel, E. R. Mitochondrial size and shape in equine skeletal muscle: A three-dimensional reconstruction study. Anat. Rec. 222, 333–339 (1988).
Kearns, C. F., McKeever, K. H. & Abe, T. Overview of horse body composition and muscle architecture: Implications for performance. Vet. J. 164, 224–234 (2002).
Tyrovolas, S. et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: A multi-continent study: Sarcopena and sarcopenic obesity in older adults. J. Cachexia Sarcopenia Muscle 7, 312–321 (2016).
Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 234, 8152–8161 (2019).
Waller, A. P., Burns, T. A., Mudge, M. C., Belknap, J. K. & Lacombe, V. A. Insulin resistance selectively alters cell-surface glucose transporters but not their total protein expression in equine skeletal muscle: Insulin resistance and glucose transport. J. Vet. Internal Med. 25, 315–321 (2011).
Banse, H. E., Frank, N., Kwong, G. P. S. & McFarlane, D. Relationship of oxidative stress in skeletal muscle with obesity and obesity-associated hyperinsulinemia in horses. Can. J. Vet. Res. 79, 329–338 (2015).
Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. WJD 6, 456 (2015).
Morgan, R. A., Keen, J. A. & McGowan, C. M. Treatment of equine metabolic syndrome: A clinical case series. Equine Vet. J. 48, 422–426 (2016).
Argo, C. M. C. G. et al. Weight loss resistance: A further consideration for the nutritional management of obese Equidae. Vet. J. 194, 179–188 (2012).
Noland, R. C. et al. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 284, 22840–22852 (2009).
Muoio, D. M. et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 15, 764–777 (2012).
Vincent, J. B. new evidence against chromium as an essential trace element. J. Nutr. 147, 2212–2219 (2017).
Jamilian, M. et al. the influences of chromium supplementation on glycemic control, markers of cardio-metabolic risk, and oxidative stress in infertile polycystic ovary syndrome women candidate for in vitro fertilization: A randomized, double-blind. Placebo-Controlled Trial. Biol. Trace Elem. Res. 185, 48–55 (2018).
Adeva-Andany, M. M., Calvo-Castro, I., Fernández-Fernández, C., Donapetry-García, C. & Pedre-Piñeiro, A. M. Significance of L-carnitine for human health. IUBMB Life 69, 578–594 (2017).
Surai, P. F. Antioxidant Action of Carnitine: Molecular Mechanisms and Practical Applications (2015).
Catandi, G. D. et al. Oocyte metabolic function, lipid composition, and developmental potential are altered by diet in older mares. Reproduction 163, 183–198 (2022).
Jocelyn, N. A., Harris, P. A. & Menzies-Gow, N. J. Effect of varying the dose of corn syrup on the insulin and glucose response to the oral sugar test. Equine Vet. J. 50, 836–841 (2018).
Frank, N. Laboratory Testing for Endocrine and Metabolic Disorders. in Interpretation of Equine Laboratory Diagnostics 401–408 (John Wiley & Sons, Inc., 2017). https://doi.org/10.1002/9781118922798.ch60.
De Laat, M. A., McGree, J. M. & Sillence, M. N. Equine hyperinsulinemia: investigation of the enteroinsular axis during insulin dysregulation. Am. J. Physiol. Endocrinol. Metab. 310, E61–E72 (2016).
Abel, E. D., O’Shea, K. M. & Ramasamy, R. Insulin resistance: Metabolic mechanisms and consequences in the heart. Arterioscler. Thromb. Vasc. Biol. 32, 2068–2076 (2012).
Thomas, D. D., Corkey, B. E., Istfan, N. W. & Apovian, C. M. Hyperinsulinemia: An early indicator of metabolic dysfunction. J. Endocr. Soc. 3, 1727–1747 (2019).
Merz, K. E. & Thurmond, D. C. Role of skeletal muscle in insulin resistance and glucose uptake. in Comprehensive Physiology (ed. Terjung, R.) 785–809 (Wiley, 2020). https://doi.org/10.1002/cphy.c190029.
Zhao, R., Jiang, S., Zhang, L. & Yu, Z. Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int. J. Mol. Med. https://doi.org/10.3892/ijmm.2019.4188 (2019).
Yu, X. & Long, Y. C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Sci. Rep. 6, 30033 (2016).
Ribas, V., García-Ruiz, C. & Fernández-Checa, J. C. Glutathione and mitochondria. Front. Pharmacol. 5 (2014).
Henry, M. L. et al. The impact of n-acetyl cysteine and coenzyme q10 supplementation on skeletal muscle antioxidants and proteome in fit thoroughbred horses. Antioxidants 10 (2021).
Das, M., Sauceda, C. & Webster, N. J. G. Mitochondrial dysfunction in obesity and reproduction. Endocrinology 162, bqaa158 (2021).
Ormazabal, V. et al. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 17, 122 (2018).
Guerra, I. M. S. et al. Mitochondrial fatty acid β-oxidation disorders: From disease to lipidomic studies—A critical review. Int. J. Mol. Sci. 23 (2022).
Schlierf, G. & Dorow, E. Diurnal patterns of triglycerides, free fatty acids, blood sugar, and insulin during carbohydrate-induction in man and their modification by nocturnal suppression of lipolysis. J. Clin. Investig. 52, 732–740 (1973).
Tortosa-Caparrós, E., Navas-Carrillo, D., Marín, F. & Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 57, 3421–3429 (2017).
Sinha, S., Haque, M., Lugova, H. & Kumar, S. The effect of omega-3 fatty acids on insulin resistance. Life 13, 1322 (2023).
Taouis, M. et al. N-3 Polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am. J. Physiol. Endocrinol. Metab. 282, e664–e661 (2002).
Bamford, N. J. Clinical insights: Treatment of laminitis. Equine Vet. J. 51, 145–146 (2019).
Respondek, F., Myers, K., Smith, T. L., Wagner, A. & Geor, R. J. Dietary supplementation with short-chain fructo-oligosaccharides improves insulin sensitivity in obese horses. J. Anim. Sci. 89, 77–83 (2011).
McGowan, C. M., Dugdale, A. H., Pinchbeck, G. L. & Argo, C. M. G. Dietary restriction in combination with a nutraceutical supplement for the management of equine metabolic syndrome in horses. Vet. J. 196, 153–159 (2013).
Manfredi, J. M., Stapley, E. D., Nadeau, J. A. & Nash, D. Investigation of the effects of a dietary supplement on insulin and adipokine concentrations in equine metabolic syndrome/insulin dysregulation. J. Equine Vet Sci. 88 (2020).
Indiveri, C. et al. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 32, 223–233 (2011).
Virmani, M. A. & Cirulli, M. The role of L-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int. J. Mol. Sci. 23 (2022).
Sangouni, A. A. et al. The effect of l-carnitine supplementation on insulin resistance, sex hormone-binding globulin and lipid profile in overweight/obese women with polycystic ovary syndrome: A randomized clinical trial. Eur. J. Nutr. 61, 1199–1207 (2022).
Xu, Y. et al. L-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 26, 333–338 (2017).
Kranenburg, L. C. et al. The effect of long-term oral L-carnitine administration on insulin sensitivity, glucose disposal, plasma concentrations of leptin and acylcarnitines, and urinary acylcarnitine excretion in warmblood horses. Vet Q. 34, 85–91 (2014).
Abdali, D., Samson, S. E. & Grover, A. K. How effective are antioxidant supplements in obesity and diabetes?. Med. Princ. Pract. 24, 201–215 (2015).
Formoso, G. et al. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab. Res. Rev. 35 (2019).
Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 78, 803–811 (2006).
Ribas, G. S., Vargas, C. R. & Wajner, M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533, 469–476 (2014).
Patel, M. S. & Korotchkina, L. G. Regulation of mammalian pyruvate dehydrogenase complex by phosphorylation: Complexity of multiple phosphorylation sites and kinases. Exp. Mol. Med. 33, 191–197 (2001).
Henneke, D. R., Potter, G. D., Kreider, J. L. & Yeates, B. F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 15, 371–372 (1983).
Carter, R. A., Geor, R. J., Burton Staniar, W., Cubitt, T. A. & Harris, P. A. Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies. Vet. J. 179, 204–210 (2009).
Gentry, L. R. et al. The relationship between body condition score and ultrasonic fat measurements in mares of high versus low body condition. J. Equine Vet. Sci. 24, 198–203 (2004).
Kane, R. A., Fisher, M., Parrett, D. & Lawrence L. M. Proceedings of the 10th Equine Nutrition and Physiology Symposium, June 11–13, 1987, the Fort Collins Marriott, Colorado State University. (Equine Nutrition and Physiology Society, 1987).
Treiber, K. H., Kronfeld, D. S., Hess, T. M., Boston, R. C. & Harris, P. A. Use of proxies and reference quintiles obtained from minimal model analysis for determination of insulin sensitivity and pancreatic beta-cell responsiveness in horses. Am. J. Vet. Res. 66, 2114–2121 (2005).
Harris, W. S., Pottala, J. V., Vasan, R. S., Larson, M. G. & Robins, S. J. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J. Nutr. 142, 1297–1303 (2012).
Metabolomics, H.-T. Methods and Protocols Vol. 1978 (Springer, 2019).
Li Puma, L. C. et al. Experimental oxygen concentration influences rates of mitochondrial hydrogen peroxide release from cardiac and skeletal muscle preparations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R972–R980 (2020).
Pesta, D. & Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol (Clifton, NJ) 810, 25–58 (2011).
Chicco, A. J. et al. Adaptive remodeling of skeletal muscle energy metabolism in high-altitude hypoxia: lessons from AltitudeOmics. J Biol. Chem. 293, 6659–6671 (2018).
Dieckmann-Schuppert, A. & Schnittler, H.-J. A simple assay for quantification of protein in tissue sections, cell cultures, and cell homogenates, and of protein immobilized on solid surfaces. Cell Tissue Res. 288, 119–126 (1997).
Source link
Share this article: